CLEO Confirms Next Generation Platform to Deliver its Ovarian Cancer Test
Highlights
CLEO selects Bio-Techne’s Ella™, a next-generation automated ELISA platform, as the immunoassay instrument for its ovarian cancer blood test
Bio-Techne (NASDAQ: TECH) is a global developer, manufacturer and supplier of high-quality reagents, analytical instruments and immunoassay technologies
The Ella™ platform can deliver materially improved assay sensitivity, precision and automation compared with conventional ELISA platforms
Binding agreement discussions well progressed with Bio-Techne for analytical validation to enable sample testing and clinical trial completion
Both parties aligned to enter long-term manufacturing supply agreement following completion of these activities.
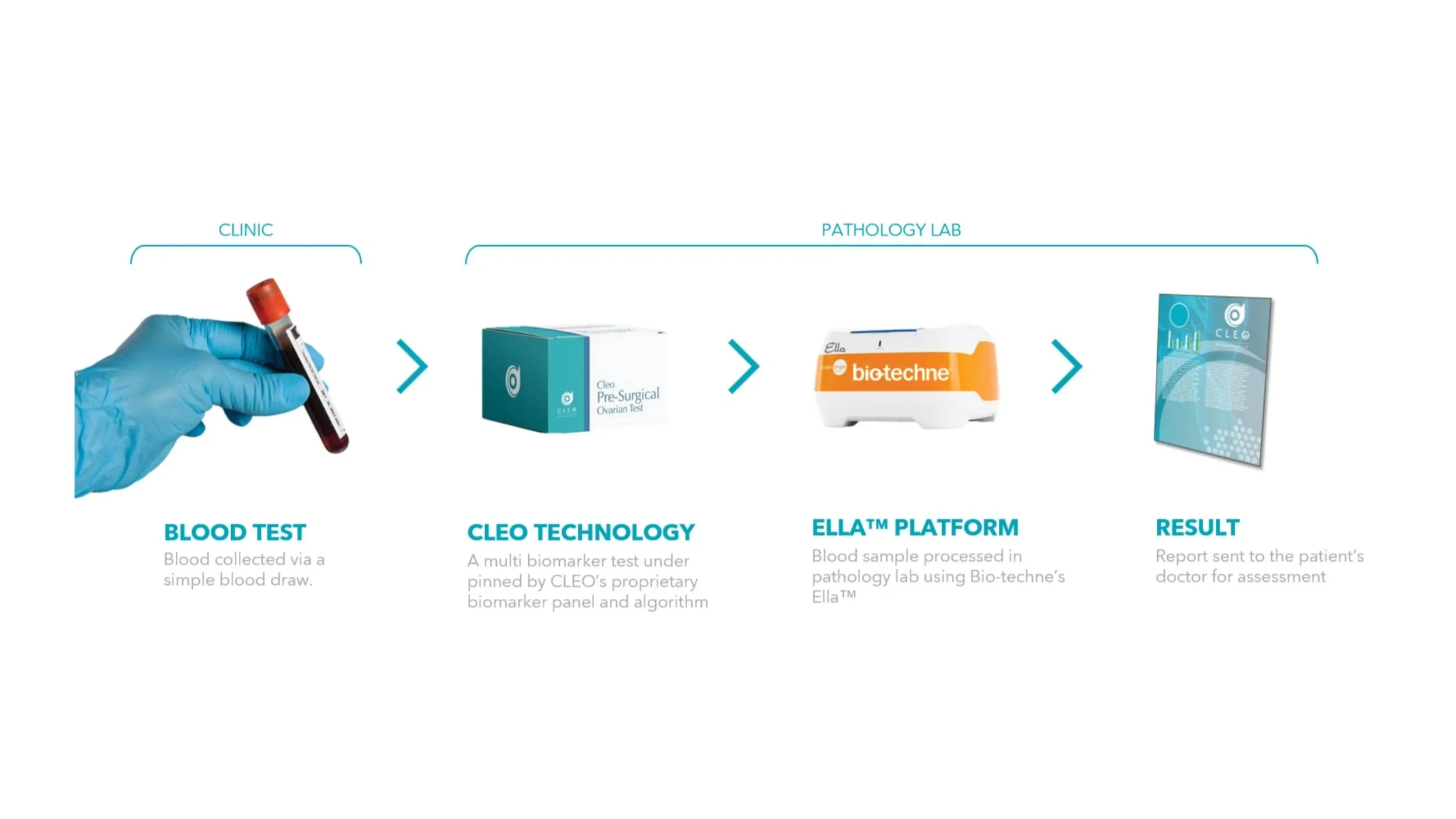
MELBOURNE, AUSTRALIA, 18th February 2026: Ovarian Cancer diagnostics company, Cleo Diagnostics Limited (ASX:COV) (CLEO or the Company) is pleased to announce that it has selected Bio-Techne’s Ella™ platform to deliver its ovarian cancer technology to market.
Next-Generation EllaTM Platform
The Ella™ platform is an automated enzyme-linked immunosorbent assay (ELISA) platform designed to deliver accurate, reproducible data with reduced manual input. Using CLEO’s test kit, patient blood samples are processed in a laboratory on the Ella™ platform. The platform uses microfluidic cartridges to measure CLEO’s proprietary biomarker panel, which form the key inputs of the algorithm underpinning the Company’s patented technology.
Key benefits of Ella™ compared to traditional ELISA include:
= Fast results (90-minute total run time versus 4+ hours per biomarker)
= Simultaneous analysis of multiple biomarkers
= Higher analytical sensitivity and precision
= Reduced manual input and improved reproducibility
= Scalability and consistency.
CLEO has been using Ella™ in-house since September last year and has confirmed the platform’s capability to deliver its ovarian cancer technology. Both parties are well progressed in discussions to enter into a binding agreement to commence analytical validation (AV) of CLEO’s test kits. To assist in this, Bio-Techne has already commenced preliminary development activities in the United States (U.S.), allowing for immediate commencement of work once formally engaged. Timeline to complete AV will be confirmed upon signing. The finished kits will then be used test the ~500 blood samples collected as part of CLEO’s pivotal clinical trial.
Importantly, these results will allow for the completion of CLEO’s clinical trial and form the primary data package of its upcoming 510(k) FDA submission. Both parties are aligned to enter into a long-term commercial supply agreement following successful completion of these activities.
Next Steps
Execute binding agreement with Bio-Techne
Commence kit manufacture for analytical validation
Analytical validation
U.S clinical trial sample testing
Analysis and results
FDA submission
About Bio-Techne
Bio-Techne Corporation (NASDAQ: TECH) is a global life sciences company providing innovative tools and bioactive reagents for research and clinical diagnostic communities. Bio-Techne products assist scientific research into biological processes and the nature and progress of specific diseases. The Company aids in drug discovery efforts and provide the means for accurate clinical tests and diagnoses. With a substantial portfolio of products, Bio-Techne generated over $1.2 billion in net sales in fiscal 2025 and has approximately 3,100 employees worldwide.